The 'Miebo' Eyedrops Directly Target Tear Evaporation
Colin Smith — September 15, 2023 — Lifestyle
References: bausch & drugstorenews
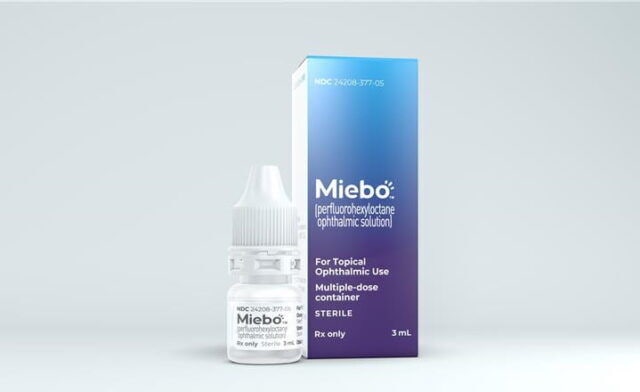
Miebo is a new prescription eye drop for the treatment of the signs and symptoms of dry eye disease (DED), a common ocular surface disorder that affects millions of Americans. Miebo is the first and only FDA-approved treatment for DED that directly targets tear evaporation, which is often caused by an altered tear lipid layer associated with Meibomian gland dysfunction (MGD). Miebo is a single ingredient, water-, preservative- and steroid-free solution that spreads quickly and comfortably on the eye. It is designed to reduce tear evaporation at the ocular surface and provide sustained improvement in both the signs and symptoms of DED.
Miebo was approved by the FDA in May 2023, based on consistent results from two pivotal phase 3 trials that enrolled more than 1,200 patients with a history of DED and clinical signs of MGD. Miebo is expected to be commercially available in the U.S. in the second half of 2023. The price of Miebo has not been announced yet, but it may vary depending on the insurance coverage and pharmacy location. Patients who are interested in Miebo should consult their eye care professional for more information and a prescription.
Image Credit: Bausch + Lomb
Miebo was approved by the FDA in May 2023, based on consistent results from two pivotal phase 3 trials that enrolled more than 1,200 patients with a history of DED and clinical signs of MGD. Miebo is expected to be commercially available in the U.S. in the second half of 2023. The price of Miebo has not been announced yet, but it may vary depending on the insurance coverage and pharmacy location. Patients who are interested in Miebo should consult their eye care professional for more information and a prescription.
Image Credit: Bausch + Lomb
Trend Themes
1. Targeted Tear-evaporation Prevention - Innovative eye drops like Miebo directly address tear evaporation associated with Meibomian Gland Dysfunction, providing a disruptive solution for dry eye disease.
2. Prescription Eye Drops for Dry Eye - Miebo marks the first FDA-approved treatment for the signs and symptoms of dry eye disease that specifically targets tear evaporation, offering a breakthrough option for patients.
3. Steroid-free Solutions for Optimal Comfort - Miebo's water-based, preservative-free, and steroid-free formula offers a rapid spread and comfortable application, revolutionizing the treatment experience for dry eye sufferers.
Industry Implications
1. Pharmaceuticals - The pharmaceutical industry can explore the development of targeted solutions like Miebo that directly address specific symptoms associated with ocular surface disorders.
2. Ophthalmology - In the field of ophthalmology, there is an opportunity to embrace innovative treatments such as Miebo that provide effective relief for dry eye disease through targeted tear evaporation prevention.
3. Healthcare - In the healthcare industry, the introduction of prescription eye drops like Miebo offers a disruptive and more specialized approach to treating dry eye disease by specifically targeting tear evaporation.
3.5
Score
Popularity
Activity
Freshness