FDA Clears Magnets for Depression
Michael Plishka — October 11, 2008 — Lifestyle
References: neuronetics & neuronetics
Depression and drugs. The two seem to always be mentioned side by side because drugs have been the primary means of treating depression. Not anymore!
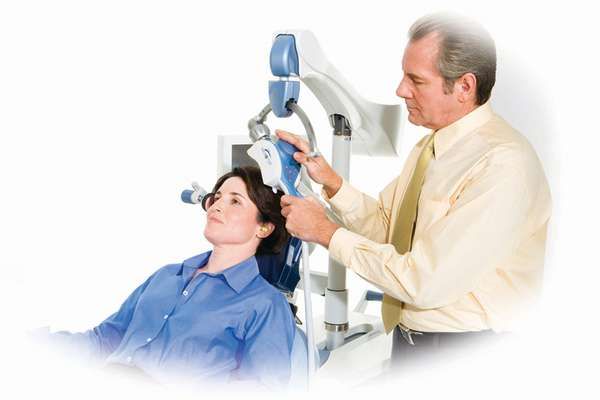
The United States Food and Drug Administration has cleared the Neuronetics NeuroStar TMS (Transcranial Magnetic Stimulation) Therapy system for the treatment of depression. This device is currently cleared for use on those people who do not respond to drug therapy.
The results of the studies using this technology have been nothing short of amazing. According to Phil Janicak, MD, a Professor of Psychiatry at Rush University-Chicago and a Principal Investigator in the NeuroStar TMS Therapy clinical trials:
In addition, this procedure is non-invasive both to the patient and to the patient’s day to day schedule. According to Neuronetics:
A video showing how this works is available here.
The United States Food and Drug Administration has cleared the Neuronetics NeuroStar TMS (Transcranial Magnetic Stimulation) Therapy system for the treatment of depression. This device is currently cleared for use on those people who do not respond to drug therapy.
The results of the studies using this technology have been nothing short of amazing. According to Phil Janicak, MD, a Professor of Psychiatry at Rush University-Chicago and a Principal Investigator in the NeuroStar TMS Therapy clinical trials:
“In the randomized controlled trial conducted for FDA clearance, NeuroStar TMS Therapy demonstrated statistically and clinically significant treatment effects...It’s particularly noteworthy that these outcomes were achieved without systemic side effects, such as weight gain and sexual dysfunction.”
In addition, this procedure is non-invasive both to the patient and to the patient’s day to day schedule. According to Neuronetics:
“TMS Therapy is a non-systemic (does not circulate in the bloodstream throughout the body) and non-invasive (does not involve surgery) form of neuromodulation which stimulates nerve cells in an area of the brain that is linked to depression, by delivering highly focused MRI-strength magnetic pulses. Patients being treated by NeuroStar TMS Therapy do not require anesthesia or sedation and remain awake and alert. It is a 40-minute outpatient procedure that is prescribed by a psychiatrist and performed in a psychiatrist’s office. The treatment is typically administered daily for 4-6 weeks.”
A video showing how this works is available here.
Trend Themes
1. Non-drug Depression Treatment - The FDA's clearance of the NeuroStar TMS Therapy system provides a disruptive innovation opportunity for non-drug therapies in the treatment of depression.
2. Neurostimulation Therapies - The use of neuromodulation to stimulate the brain cells linked to depression provides a disruptive innovation opportunity for neurostimulation therapies.
3. Non-invasive Medical Procedures - The non-invasive nature of the NeuroStar TMS Therapy provides a disruptive innovation opportunity for the development of more non-invasive medical procedures in the treatment of depression.
Industry Implications
1. Medical Device - The clearance by the FDA of the NeuroStar TMS Therapy system provides a disruptive innovation opportunity for medical device manufacturing companies in the development of neurostimulation devices for the treatment of depression.
2. Pharmaceuticals - The increasing prevalence of drug-resistant depression provides a disruptive innovation opportunity for pharmaceutical companies in the development of non-drug therapies for the treatment of depression.
3. Mental Health Services - The development of non-drug therapies, such as the NeuroStar TMS Therapy, provides a disruptive innovation opportunity for mental health service providers in the treatment of depression.
1.7
Score
Popularity
Activity
Freshness